Abstract
Background: Acalabrutinib is a highly selective Bruton tyrosine kinase inhibitor approved for relapsed/refractory mantle cell lymphoma (MCL). We investigated safety and efficacy of chemotherapy-free induction with combination acalabrutinib, venetoclax, and rituximab (AVR) in treatment-naive patients (pts) with MCL to eliminate intensive chemoimmunotherapy exposure and reduce associated toxicity and complications. We previously reported 90% complete response (CR) and 75% molecular response rates at 20.5 mo median follow-up (ASH 2021). We present updated safety and efficacy results at 2 y median follow-up.
Methods: Adults with treatment-naive MCL received AVR in this open-label, single-arm, multicenter phase 1b study (NCT02717624). Pts received A+R for six 28-d cycles with maintenance R for up to 2 y for responders and oral A continuously until progressive disease (PD) or undue toxicity. V was started at cycle 2 with standard 5-wk ramp-up to 400 mg/d and continued for 2 y. Primary endpoint was safety. Secondary endpoints were overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS). Treatment response was assessed by clinical examination, computed tomography (CT), and positron emission tomography (PET) by Lugano criteria, with minimal residual disease (MRD) assessment by clonoSEQ assay in peripheral blood (at partial response [PR], CR, and PD). Bone marrow (BM) was assessed at screening and CR.
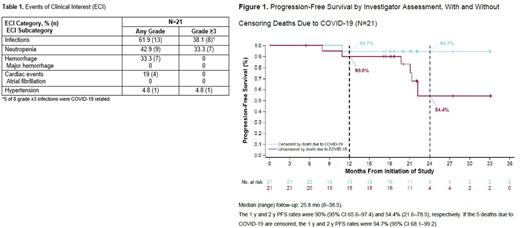
Results: Twenty-one pts were enrolled (median age 66 y [51-85]; Ann Arbor stage IV disease, 90.5% [n=19]; initial BM involvement, 71.4% [n=15]; high-risk simplified MIPI score, 19% [n=4]; blastoid histology, 4.8% [n=1]; pleomorphic variant, 0%; bulky disease >5 cm or >10 cm, 33.3% [n=7] and 4.8% [n=1]; Ki-67 proliferation index ≥30%, 47.6% [n=10]). At data cutoff (March 29, 2022), 15 (71.4%) pts remained on study; 6 (28.6%) had discontinued study, due to death (5 [23.8%]) or start of new therapy (1 [4.8%]). Eleven (52.4%) pts remained on at least 1 study drug and 7 pts (33.3%) discontinued all 3 drugs: 2 due to PD and 5 due to COVID-19 death. Ten pts (47.6%) discontinued A (adverse event [AE]=5, investigator's decision [ID]=3, PD=2); 17 discontinued V (completed regimen=9, PD=2, AE=4, ID=1, other=1); and 17 discontinued R (completed regimen=10, PD=2, AE=4, ID=1) All 21 pts (100%) completed the 6 initial cycles of treatment (induction) per protocol. Most common any-grade AEs in ≥20% of pts were diarrhea (15 [71.4%]), headache (11 [52.4%]), fatigue (10 [47.6%]), and neutropenia (8 [38.1%]). Grade 3/4 AEs were reported in 12 (57.1%) pts, most commonly (>1 pt) neutropenia (7 [33.3%]) and pneumonia (3 [14.3%]). Serious AEs (SAEs) occurred in 12 (57.1%) pts. SAEs in ≥2 pts were COVID-19 (5 [23.8%]), pneumonia (3 [14.3%]), and pyrexia (2 [9.5%]). Overall, 7 pts had COVID-19; 6 (28.6%) cases were treatment-emergent (up to 30 days after last dose) and 4 (19.0%) pts died. The seventh pt with COVID-19 died outside the treatment-emergent period. Table 1 shows events of clinical interest. At data cutoff, ORR was 100% (n=21), CR rate was 90% (n=19), and PR rate was 10% (n=2) by PET/CT. By Lugano, CR rate was 71% (n=15) since 4 pts were missing BM biopsy to confirm CR. Median (range) time to initial response and best response were 2.8 (0.7-5.6) and 3.0 (0.7-18.3) mo, respectively. Median DOR was not reached even after censoring COVID-19 deaths. With a median follow-up of 25.8 (8-38.5) mo, the median PFS and OS were not reached. PFS rates at 1 y and 2 y were 90% (95% CI 65.6-97.4) and 54.4% (21.6-78.5), respectively (both were 94.7% [68.1-99.2] after censoring COVID-19 deaths) (Figure 1). OS rates at 1 y and 2 y were 95.2% (95% CI 70.7-99.3) and 74.1% (48.4-88.3), respectively (both were 100% after censoring). In total, 5 pts died, all from COVID-19. MRD data were available for 12 pts (57.1%) at cycle 6 (all MRD negative at 10-6) and 14 pts (66.7%) at cycle 12 (12 negative at 10-6; 14 negative at 10-4). Fourteen of 16 (87.5%) evaluable pts had MRD negativity (10-6) at least once during treatment. Among the 15 pts who achieved CR by PET/CT, 13 (86.7%) were MRD negative at 10-6 and 1 pt was MRD negative at 10-5 at least once during treatment; 1 pt had no follow-up MRD assessments.
Conclusions: AVR combination is well tolerated and induces durable and deep clinical and molecular responses in treatment-naive MCL pts. This study strongly supports further clinical investigation of AVR for treatment-naive MCL.
Disclosures
Wang:Mumbai Hematology Group: Honoraria; Moffit Cancer Center: Honoraria; Meeting Minds Experts: Honoraria; Medscape: Honoraria; TS Oncology: Honoraria; Eastern Virginia Medical School: Honoraria; Dava Oncology: Honoraria; Clinical Care Options: Honoraria; Anticancer Association: Honoraria; Vincerx: Research Funding; Molecular Templates: Research Funding; Genmab: Research Funding; Celgene: Research Funding; Acerta Pharma: Honoraria, Research Funding; VelosBio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Pepromene Bio: Consultancy; Oncternal: Consultancy, Research Funding; Miltenyi Biomedicine GmbH: Consultancy, Honoraria; Loxo Oncology: Consultancy, Research Funding; Lilly: Consultancy, Research Funding; Leukemia & Lymphoma Society LLC: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Research Funding; BGICS: Honoraria; CAHON: Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; LLC TS Oncology: Honoraria; OMI: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria; Practice Point Communications (PPC): Honoraria; First Hospital Zhejiang University: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; InnoCare: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Epizyme: Consultancy, Honoraria; DTRM Biopharma (Cayman) Limited: Consultancy; Deciphera: Consultancy; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; CStone: Consultancy; BioInvent: Consultancy, Honoraria, Research Funding. Robak:Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; OctaPharma: Honoraria, Research Funding; Regeneron: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Maddocks:Morphosys: Consultancy; Lilly: Consultancy; Kite: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Gilead: Consultancy; Genentech: Consultancy; Genmab: Consultancy; Celgene: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Acerta: Consultancy; Abbvie: Consultancy; BMS: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Pfizer: Research Funding; Pharmacyclics: Consultancy, Research Funding. Phillips:Incyte: Consultancy; Pharmacyclics/Janssen: Honoraria; Celgene: Consultancy; Eli Lilly: Consultancy; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Xencor: Consultancy; Abbvie: Consultancy, Research Funding; ADCT: Consultancy; Kite/Gilead: Consultancy; Genmab: Consultancy; BMS: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; TG Therapeutics: Consultancy; Curis: Consultancy; Epizyme: Consultancy; Lymphoma & Myeloma Connect: Honoraria; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Smith:Portola Pharmaceuticals: Research Funding; Nanjing Pharmaceuticals Co., Ltd.: Research Funding; MorphoSys: Research Funding; Kymera Therapeutics: Research Funding; KITE pharma: Consultancy; Karyopharm: Consultancy; Bayer: Research Funding; Viracta Therapeutics: Research Funding; Abbvie: Consultancy; Numab Therapeutics AG: Consultancy; Merck Sharp and Dohme Corp.: Research Funding; Incyte Corporation: Consultancy, Research Funding; Genentech: Research Funding; Epizyme: Consultancy; Enterome: Research Funding; De Novo Biopharma: Research Funding; Beigene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding. Calvo:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Wun:Astrazeneca: Current Employment. Munugalavadla:Gilead: Current equity holder in publicly-traded company; AstraZeneca: Current Employment. Jurczak:Janssen: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Mei Pharma: Research Funding; Lilly: Consultancy, Research Funding; Takeda: Research Funding; Roche: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Bayer: Research Funding; Celgene: Research Funding; TG Therapeutics: Research Funding; Loxo Oncology: Consultancy, Research Funding; Sandoz: Consultancy, Research Funding; Merck: Research Funding; Morphosys: Research Funding; Novo Nordisk: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal